STAKEHOLDERS in the health sector have welcomed the introduction of the pre-exposure prophylaxis (PrEP) injectable drug saying it will boost the fight against HIV/Aids.
National Aids Council chief executive officer, Bernard Madzima, said this was a major development in the provision of Anti-Retroviral Therapy services.
“We welcome the scientific development which has taken place in having an injectable ARV, in this case, which is Cabotegravir,” Madzima said.
“This is the start but as you then look at who is getting it and at what cost, as a country, our policies and guidelines need to be guided.”
“If we are to say we have policies and guidelines, in terms of using Cabotegravir as PrEP, we need to have consensus on whom we are targeting.”
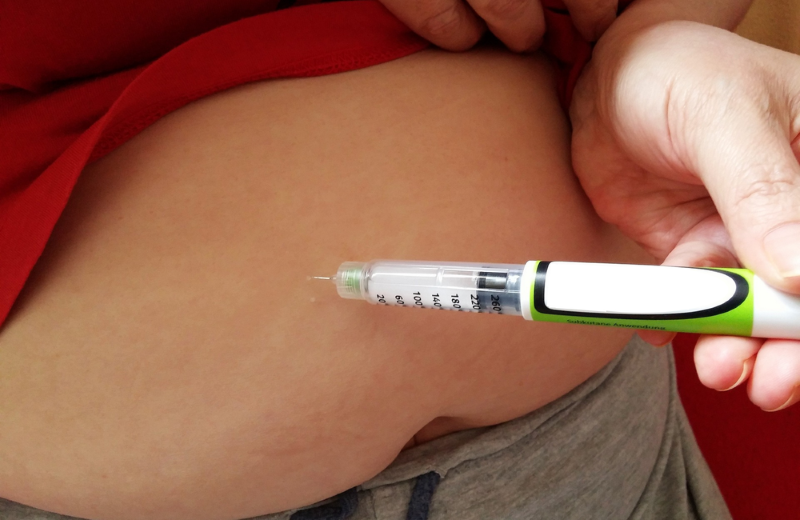
Zimbabwe has introduced the Cabotegravir long-acting (CAB-LA), an injectable pre-exposure prophylaxis drug as another HIV preventive measure.
The injectable PrEP becomes the third PrEP product to be introduced in the fight against HIV.
Madzima said the cost of the injectable drug needed to be looked at.
- Zim pilots new HIV drug
- TB preventive treatment: Need for choice
- Experts welcome rollout of HIV injectable drug
Keep Reading
“We understand that a single injection costs around US$150 and so it becomes an issue of affordability to those who want to take it in the private sector,” he said.
“But as a government programme, we are still to decide or to agree on the policy or guidelines which we need to check.”
Community Working Group on Health executive director Itai Rusike said it was encouraging that Zimbabwe had become one of the first countries in Africa to approve the drug.
“The government should now make sure that drug is widely available and easily accessible to all our health institutions and to all those that need it without facing financial hurdles,” he said.
“PrEP dramatically reduces the risk of HIV acquisition for women as well as men. “We are aware that for more than a decade the World Health Organisation has recommended that pregnant and breastfeeding women at risk of HIV should be offered PrEP, and the people of Zimbabwe should embrace this new development in order to reduce the risk of HIV infection.”
He urged the government to make sure that people are provided with more information about the injectable PrEP to help them to make informed decisions.
The country's fight against HIV has seen Aids-related deaths fall from an estimated 130 000 in 2002 to 20 000 in 2021.










